Diferencia entre revisiones de «Quimera Rosa»
(→Bitacora Residencia) |
(→Día 2: Experiment. Campthotecin treatment to induce apoptosis in a dose-dependent manner) |
||
| Línea 109: | Línea 109: | ||
| − | '''Protocol''' | + | === '''Protocol''' === |
| + | |||
Jurkat cells are maintained in the culture medium RPMI, supplemented with 10% fetal bovine serum (FBS), 1% Pen/Strep and Glutamax. | Jurkat cells are maintained in the culture medium RPMI, supplemented with 10% fetal bovine serum (FBS), 1% Pen/Strep and Glutamax. | ||
| Línea 115: | Línea 116: | ||
1. Prepare cells in fresh RPMI medium at a concentration of 1 x 106 cells/mL in desired tubes: | 1. Prepare cells in fresh RPMI medium at a concentration of 1 x 106 cells/mL in desired tubes: | ||
(we prepare 7 tubes with 1 x 106 cells each) | (we prepare 7 tubes with 1 x 106 cells each) | ||
| − | + | *Check cells at the microscope | |
| − | + | *Collect the cells from the flask in a falcon and centrifuge at 300g for 6 min at room temperature | |
| − | Collect the cells from the flask in a falcon and centrifuge at 300g for 6 min at room temperature | + | *Discard supernatant and add 10 mL of medium |
| − | Discard supernatant and add 10 mL of medium | + | *Count cells |
| − | Count cells | + | *Add amount of medium necessary to have 1 x 106 cells/mL |
| − | Add amount of medium necessary to have 1 x 106 cells/mL | + | *Add 1mL to each corresponding tube (1 x 106 cells) |
| − | Add 1mL to each corresponding tube (1 x 106 cells) | + | |
A 10-mM stock solution of Camptothecin was prepared in dimethyl sulfoxide (DMSO) and diluted to final concentrations of 0.1, 1 ,10 and 50 μM into RPMI medium. | A 10-mM stock solution of Camptothecin was prepared in dimethyl sulfoxide (DMSO) and diluted to final concentrations of 0.1, 1 ,10 and 50 μM into RPMI medium. | ||
2. Add an appropriate amount of 10 mM Camptothecin to the cell suspension(1x10^6) to achieve a final concentration of 0, 0.1, 1.0 and 10 μM: | 2. Add an appropriate amount of 10 mM Camptothecin to the cell suspension(1x10^6) to achieve a final concentration of 0, 0.1, 1.0 and 10 μM: | ||
| + | *Centrifuge the tubes at 300g for 6 min at room temperature | ||
| + | *Discard supernatant | ||
| − | |||
| − | |||
Add 1mL of Camptothecin 50 μM to tube ‘50 μM’ and resuspend cells pipetting | Add 1mL of Camptothecin 50 μM to tube ‘50 μM’ and resuspend cells pipetting | ||
Add 1mL of Camptothecin 10 μM to tube ‘10 μM’ and resuspend cells pipetting | Add 1mL of Camptothecin 10 μM to tube ‘10 μM’ and resuspend cells pipetting | ||
| Línea 134: | Línea 134: | ||
Add 1mL of Camptothecin 0.1 μM to tube ‘0.1 μM’ and resuspend cells pipetting | Add 1mL of Camptothecin 0.1 μM to tube ‘0.1 μM’ and resuspend cells pipetting | ||
Add 1mL of RPMI medium to tube ‘Untreated’ and resuspend cells pipetting | Add 1mL of RPMI medium to tube ‘Untreated’ and resuspend cells pipetting | ||
| − | |||
3. Incubate cells at 37°C, 5% CO2 for 4 hours. | 3. Incubate cells at 37°C, 5% CO2 for 4 hours. | ||
| − | + | *Apoptotic cells staining with Annexin V and Propidium Iodide | |
| − | Apoptotic cells staining with Annexin V and Propidium Iodide | + | |
| − | + | ||
4. Centrifuge the tubes at 300g for 6 min at room temperature and discard supernatant | 4. Centrifuge the tubes at 300g for 6 min at room temperature and discard supernatant | ||
5. Wash two times with PBS: | 5. Wash two times with PBS: | ||
| − | + | *Resuspend cells with 1mL PBS | |
| − | Resuspend cells with 1mL PBS | + | *Centrifuge the tubes at 300g for 6 min at room temperature |
| − | Centrifuge the tubes at 300g for 6 min at room temperature | + | *Discard the supernatant |
| − | Discard the supernatant | + | *Resuspend cells with 1mL PBS |
| − | Resuspend cells with 1mL PBS | + | *Centrifuge the tubes at 300g for 6 min at room temperature |
| − | Centrifuge the tubes at 300g for 6 min at room temperature | + | *Discard the supernatant |
| − | Discard the supernatant | + | |
| − | + | ||
6. Resuspend cells with 1mL of 1X Binding buffer | 6. Resuspend cells with 1mL of 1X Binding buffer | ||
Revisión actual del 19:53 12 sep 2017
- TransPlant *: Green is the new Red es un proyecto transdisciplinar de hibridación planta/humano/animal/máquina que Quimera Rosa ha iniciado en 2016 y que se va a desarrollar a lo largo de los próximos años. TransPlant es un proyecto de bio-arte basado en la auto-experimentación así que un proceso que compromete un cuerpo en una transición interbioformae (1). TransPlant pone en dialogo disciplinas como arte, filosofía, biología, ecología, física, botánica, medicina, enfermería, farmacología y electrónica. Mediante diversas prácticas de bio-hacking, TransPlant se inscribe en los debates en curso sobre la noción de Antropoceno, desde una perspectiva no basada sobre “el excepcionalismo humano y el individualismo metodológico” (2), sino que aborda el mundo y sus habitantes como el producto de procesos cyborg, de devenir con (3), de simpoiesis (4).
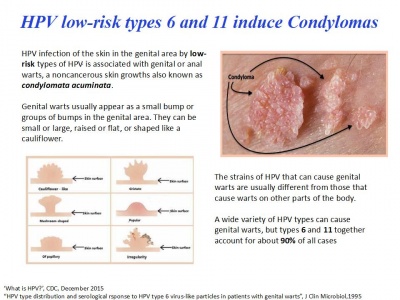
El desarrollo de este proyecto está basado sobre la interacción entre diferentes ejes destinados a producir cambios de subjetividad y desconstruir diferentes tipos de narrativas que presentan el cuerpo como una unidad. Estos ejes son por el momento: hibridación de sangre humana con clorofila con un protocolo regular de inyecciones por intravenosa, traducción externa con tatuajes de clorofila, implantación de un chip electrónico RFID donde estarán almacenados los datos del proceso y presentando el cuerpo como una somateca (5), desarrollo y conexión al cuerpo de sensores propios a las plantas (nivel de acidez del entorno, frecuencias electro-magnéticas especificas…) y feed-back con la actividad corporal, auto-experimentación medica sobre condylomata acuminata, constitución de una base de datos publica open-source de los experimentos.
http://quimerarosa.net/wiki
http://quimerarosa.net/wiki/index.php/Residencia_PRBB
Contenido
Bitacora Residencia
Como parte de la residencia Prototyp_ome hemos estado dos días trabajando en el Departamento de Biología de la Infección (Andreas Meyerhans y Jordi Argilaguet) del Parque de Resercha Biomédica de Barcelona .
Workshop dado por Valentina Casella (Biología de la infección) ||| Con la traducción científica, el acompañamiento y las risas de Nuria Conde (DiyBio Barcelona)
Tanto las imágenes explicativas como los protocolos nos fueron dados durante el workshop. Dejamos aquí solo una selección.
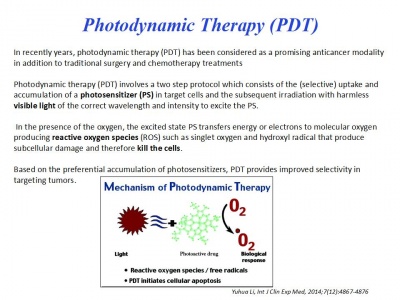
Acerca del HPV y la Terapia Fotodinámica
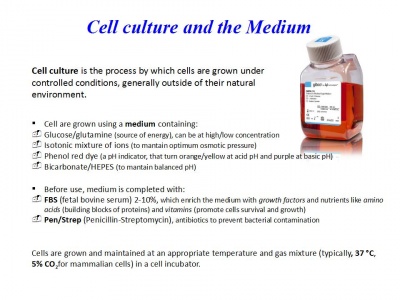
Dia 1: Working with cell cultures: Cells passage (splitting) and counting
Protocol
1) Prepare a new flask and put PBS, Trypsin, Medium (RPMI/DMEM+ 2-10%FBS) in the bath at 37°C for 10 min 2) Check cells at the Microscope
(In case of adherent cells ex. MC57)
3) Remove old medium from the flask 4) Wash with PBS two times 5) Add 1.5 mL of Trypsin for 1-2 min and put cells in the incubator at 37°C 6) Then hit gently the flask on the side to make it easier for the cells to detatch 7) Add medium to the flask and recollect cells in a falcon 8) Centrifuge cells at 600G for 6 minutes at room temperature 9) Remove supernatant 10)Resuspend cells in a falcon with new medium ( ex. 5 mL) 11) Count cells (see next protocol) 12) Add to the cells the amount of medium required in order to have them at the concentration desired (for ex. 1x106/mL) 13) Prepare a new flask with fresh medium and add cells
(In case of cells in suspension ex. Jurkat)
3) Recollect medium from the flask in a falcon 4) Centrifuge cells at 600G for 6 minutes at room temperature 5) Remove supernatant 6) Resuspend cells in new medium ( ex. 5 mL) 7) Count cells 8) Add to the cells the amount of medium required in order to have them at the concentration desired (for ex. 1x106/mL) 9) Prepare a new flask with fresh medium and add cells
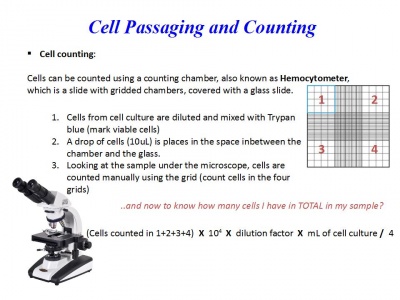
Cell counting
Cells can be counted using a counting chamber, also known as Hemocytometer, which is a slide with gridded chambers, covered with a glass slide.
1. Cells from cell culture are diluted and mixed with Trypan blue (mark viable cells):
- prepare a eppendorf with 90 μL medium+10 μL cells and mix (dilution 1:10)
- take 10 μL from this dilution and mix in a new eppendorf with 10 μL L of Trypan blue (dilution 1:2)
So now we have a total dilution factor of 20 (10x2)
2. Prepare the hemocytometer with the slide of glass placed over it and transfer 10 μL from the last mix, in the space in-between the chamber and the glass.
3. Looking at the sample under the microscope, cells are counted manually using the grid (count cells in the four grids)
Counting formula
(Cells counted in 1+2+3+4) X 104 X dilution factor X mL of cell culture / 4
Archivo:Residencia PRBB dia 1 Quimera Rosa 048.JPG Archivo:Residencia PRBB dia 1 Quimera Rosa 039.JPG
Archivo:Residencia PRBB dia 1 Quimera Rosa 054.JPG Archivo:Residencia PRBB dia 1 Quimera Rosa 017.JPG
Archivo:Residencia PRBB dia 2 Quimera Rosa 101.JPG Archivo:Residencia PRBB dia 1 Quimera Rosa 026.JPG
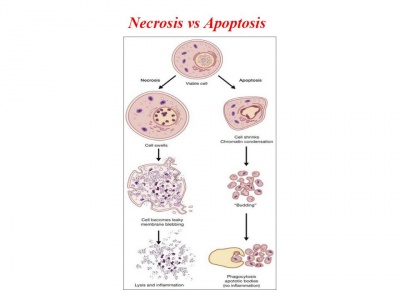
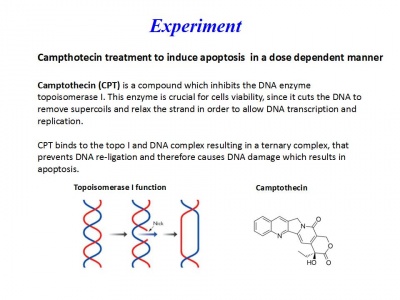
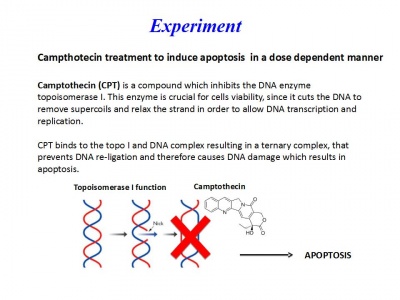
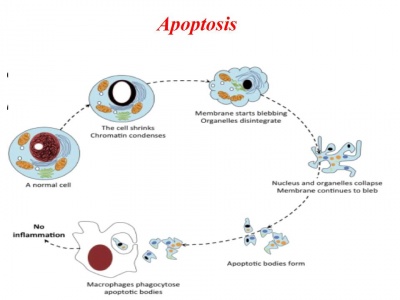
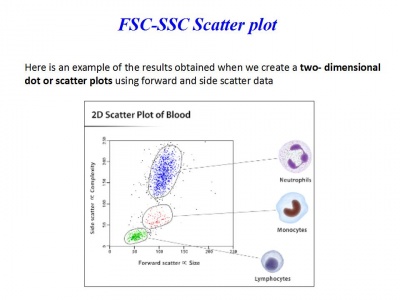
Día 2: Experiment. Campthotecin treatment to induce apoptosis in a dose-dependent manner
Protocol
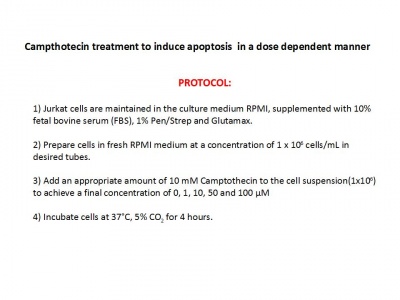
Jurkat cells are maintained in the culture medium RPMI, supplemented with 10% fetal bovine serum (FBS), 1% Pen/Strep and Glutamax.
1. Prepare cells in fresh RPMI medium at a concentration of 1 x 106 cells/mL in desired tubes: (we prepare 7 tubes with 1 x 106 cells each)
- Check cells at the microscope
- Collect the cells from the flask in a falcon and centrifuge at 300g for 6 min at room temperature
- Discard supernatant and add 10 mL of medium
- Count cells
- Add amount of medium necessary to have 1 x 106 cells/mL
- Add 1mL to each corresponding tube (1 x 106 cells)
A 10-mM stock solution of Camptothecin was prepared in dimethyl sulfoxide (DMSO) and diluted to final concentrations of 0.1, 1 ,10 and 50 μM into RPMI medium.
2. Add an appropriate amount of 10 mM Camptothecin to the cell suspension(1x10^6) to achieve a final concentration of 0, 0.1, 1.0 and 10 μM:
- Centrifuge the tubes at 300g for 6 min at room temperature
- Discard supernatant
Add 1mL of Camptothecin 50 μM to tube ‘50 μM’ and resuspend cells pipetting Add 1mL of Camptothecin 10 μM to tube ‘10 μM’ and resuspend cells pipetting Add 1mL of Camptothecin 1 μM to tube ‘1 μM’ and resuspend cells pipetting Add 1mL of Camptothecin 0.1 μM to tube ‘0.1 μM’ and resuspend cells pipetting Add 1mL of RPMI medium to tube ‘Untreated’ and resuspend cells pipetting
3. Incubate cells at 37°C, 5% CO2 for 4 hours.
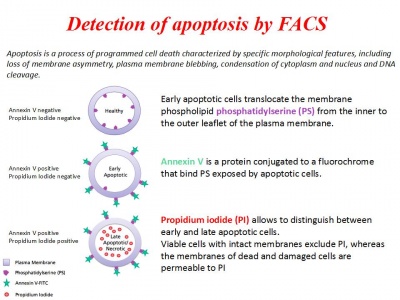
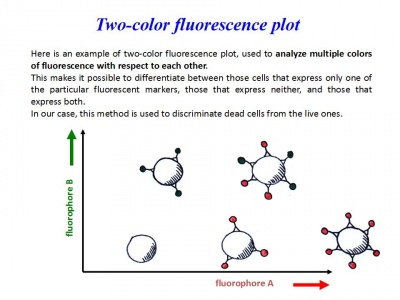
- Apoptotic cells staining with Annexin V and Propidium Iodide
4. Centrifuge the tubes at 300g for 6 min at room temperature and discard supernatant
5. Wash two times with PBS:
- Resuspend cells with 1mL PBS
- Centrifuge the tubes at 300g for 6 min at room temperature
- Discard the supernatant
- Resuspend cells with 1mL PBS
- Centrifuge the tubes at 300g for 6 min at room temperature
- Discard the supernatant
6. Resuspend cells with 1mL of 1X Binding buffer
7. Transfer 300 μL of this solution to new FACS tubes
8. Add 15 μL of FITC Annexin V and 15 μL of Propidium Iodide (PI)
9. Vortex and incubate at room temperature for 15 minutes in the dark!
10. Add 400 μL of 1X Binding buffer to each tube
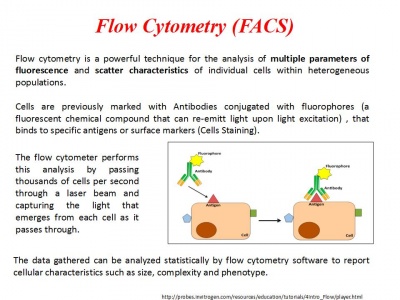
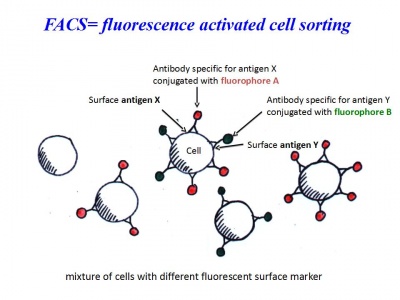
Analyse at FACS within 1 hour!
Archivo:Residencia PRBB dia 2 Quimera Rosa 002.JPG Archivo:Residencia PRBB dia 2 Quimera Rosa 051.JPG
Archivo:Residencia PRBB dia 2 Quimera Rosa 021.JPG Archivo:Residencia PRBB dia 2 Quimera Rosa 029.JPG
Archivo:Residencia PRBB dia 2 Quimera Rosa 041.JPG Archivo:Residencia PRBB dia 2 Quimera Rosa 014.JPG
Archivo:Residencia PRBB dia 2 Quimera Rosa 103.JPG Archivo:Residencia PRBB dia 2 Quimera Rosa 121.JPG
Células tratadas por las Quimeras en proceso de apoptosis (induciendo suicidio en masa)
Archivo:Quimera-Layout.jpg Archivo:Quimera-Layout.png